What are the three requirements in the Huckel rule for aromaticity and Use the Buckle rule to show that Benzene is aromatic: Synthetic Chemistry Course Work, UCD, Ireland
| University | University College Dublin (UCD) |
| Subject | Synthetic Chemistry |
Question 1
A) What are the three requirements in the Huckel rule for aromaticity?
B) Use the Buckle rule to show that Benzene is aromatic.
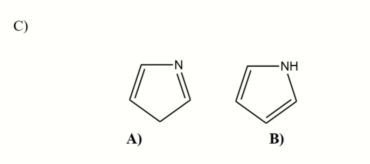
Identify which of the two structures is aromatic? For both explain to the masons why it is either aromatic or nonaromatic?
Question 2
A) Using Woodward-Fieser’s rules, calculate wavelengths of maximum UV absorption for the following compounds:
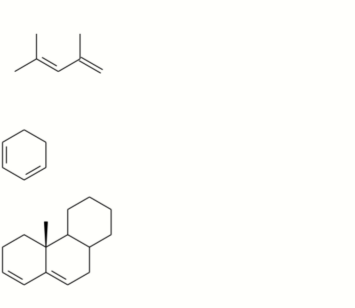
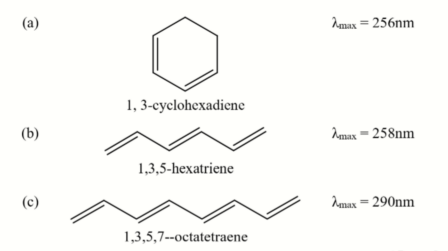
B) Define the terms ‘HOMO’ and `LUM0′ and comment on the observed UV-vis trend of (a) — (c) below:
C) Discuss the addition of ethylene to I,3-butadiene in terms of the Diets-Alder cycloaddition and using two examples explain how the presence of other conjugated unsaturated groups on the dienophile can affect the reaction.
Get Solution of this Assessment. Hire Experts to solve this assignment for you Before Deadline.
At Irelandassignments.ie Our professional team of chemistry experts possesses a profound knowledge base of Synthetic Chemistry which enables us to provide you with the best quality CHEM20050 Medicinal Chemistry And Chemical Biology (Level 2) Assignment with detailed information. Besides, we can assist you with any kind of childcare assignment help at a very low price.
